
TNF Alpha Inhibitors Market By Drug Class (Adalimumab, Certolizumab Pegol, Etanercept, Golimumab, Infliximab), By Pipeline Analysis (Phase I, Phase II, Phase III), By Regions (North America, Europe, Asia Pacific, Rest of the World) – Global Forecast up to 2025
- August, 2019
- Domain: Healthcare - Pharmaceuticals
- Get Free 10% Customization in this Report
This market research report includes a detailed segmentation of the global TNF alpha inhibitors market by drug class (adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab), pipeline analysis (phase I, phase II, and phase III), and regions (North America, Europe, Asia Pacific, and Rest of the World).
Overview of the Global TNF Alpha Inhibitors Market Research:
Infoholic’s market research report predicts that the global TNF alpha inhibitors market will grow at a CAGR of 3.6% during the forecast period 2019–2025. TNF inhibitors, also known as TNF blockers, anti-TNF drugs, and biologic therapies, are a cluster of medications used to treat inflammatory conditions, including rheumatoid arthritis, juvenile arthritis, psoriatic arthritis, ankylosing spondylitis, inflammatory bowel disease (Crohn’s and ulcerative colitis), and psoriasis. The drugs decrease inflammation and can stop disease development by targeting an inflammation-causing substance, tumor necrosis factor (TNF).
The market is witnessing the loss of patents in Europe for various blockbuster drugs, resulting in the emergence of biosimilars. In April 2019, Eticovo – a biosimilar from Samsung Bioepis to Amgen’s Enbrel (etanercept), received FDA approval. In November 2018, FDA approved Hyrimoz developed by Sandoz, a biosimilar to AbbVie’s blockbuster drug Humira, which will be launched in the US market by 2023. Currently, only five approved TNF inhibitors, i.e., adalimumab, certolizumab, etanercept, golimumab, and infliximab, control the market. The wide application of these drugs for numerous autoimmune diseases ensures constant growth of the market during the forecast period 2019–2025.
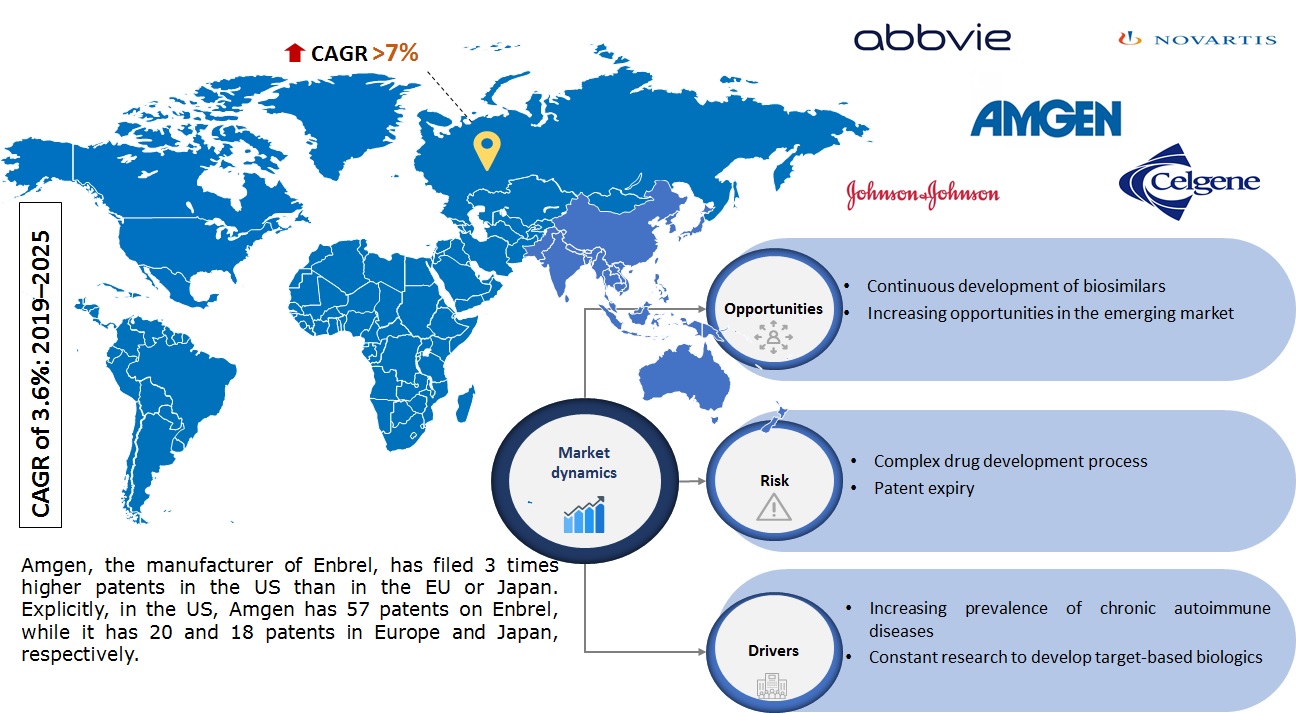
Key players operating in this field, i.e., AbbVie Inc., Amgen Inc., Johnson & Johnson, and Celgene Corporation, are generating the majority of the revenue in the global TNF alpha inhibitors market.
According to Infoholic Research analysis, North America accounted for the largest share of the global TNF alpha inhibitors market in 2018 and will retain a high position during the forecast period. North America is followed by Europe, with a market share of more than 20% in 2018.
By Drug Class:
- Adalimumab
- Certolizumab Pegol
- Etanercept
- Golimumab
- Infliximab
In 2018, the adalimumab segment accounted for the largest share and is expected to grow at a mid-single-digit CAGR during the forecast period. Increased clinical efficacy and high preference by physicians for the treatment of severe rheumatic diseases made the segment the largest shareholder in 2018. Golimumab is expected to grow at a high CAGR during the forecast period 2019–2025.
Pipeline Analysis:
- Phase I
- Phase II
- Phase III
Many companies are making huge investments to develop products and enter the market as soon as biologics lose their patents. Among various TNF alpha inhibitors, adalimumab has the highest number of ongoing clinical trials, followed by certolizumab pegol.
By Regions:
- North America
- Europe
- Asia Pacific
- Rest of the World
Based on geography, North America dominated the market in 2018 with a market share of over 60% and is expected to maintain this position during the forecast period. Europe is projected to grow at a high CAGR during the forecast period. The increasing acceptance of biosimilars and increasing investments in the R&D of new drugs make Europe the fastest-growing region during the forecast period.
Global TNF Alpha Inhibitors Market Research Competitive Analysis: The market is growing at a steady rate, i.e., at a CAGR of 3.6% during the forecast period 2019–2025. New drug launches, product approvals, strategic partnerships, and collaborations are among the significant strategies adopted by market leaders to maintain their leadership position. For instance, in July 2019, the US FDA approved HADLIMA by Samsung Bioepis, a biosimilar referencing HUMIRA, to treat juvenile idiopathic arthritis, adult Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, rheumatoid arthritis, and plaque psoriasis. In March 2019, the US FDA approved Cimzia injection to treat non-radiographic axial spondyloarthritis (nr-axSpA) in adults, with detached signs of swelling making it the first-ever FDA approved treatment fulfilling the unmet needs for nr-axSpA; and in May 2018, the drug was approved for the treatment of moderate-to-severe plaque psoriasis.
The rising incidence of chronic autoimmune diseases has led to the increasing launch of biosimilars globally. In May 2019, Fresenius Kabi launched biosimilar adalimumab IDACIO in Germany. In May 2019, Janssen launched Simponi Autoinjector in Japan, and the drugs are distributed by Mitsubishi Tanabe. In addition, other leading vendors are focusing on hugely investing in R&D activities to develop new drugs to obtain a high share in the market.
Key Vendors:
- AbbVie Inc.
- Novartis AG
- Amgen Inc.
- Johnson & Johnson
- Celgene Corporation
- Celltrion Healthcare
- Samsung Bioepis Co. Ltd.
- Apogenix
- 3SBIO Inc.
- Shanghai CP Guojian Pharmaceutical Co. Ltd.
Key Competitive Facts:
- UCB Biopharma S.P.R.L. is conducting a phase 3 clinical trial in 15 centers in Germany to study “Efficacy and safety of certolizumab pegol (CZP) versus active comparator and placebo in subjects with Plaque Psoriasis (PSO) (CIMPACT)”.
- Globally, over 95% of the clinical trials of certolizumab are conducted by UCB Pharma.
- In July 2018, Mylan made a patent license agreement with AbbVie over Mylan’s proposed Humira biosimilar. Under the terms of the agreement, AbbVie will grant Mylan exclusive licenses on Humira’s intellectual properties on July 31, 2023 in the US and other countries excluding Europe.
Benefits – The report provides complete details about the sub-segment of the global TNF alpha inhibitors market. Thus, the key stakeholders can know about the major trends, drivers, investments, vertical player’s initiatives, and government initiatives toward the pharmaceuticals segment in the upcoming years along with details of the pureplay companies entering the market. Moreover, the report provides details about the major challenges that are going to impact the market growth. Additionally, the report gives complete details about the key business opportunities to key stakeholders in order to expand their business and capture the revenue in specific verticals, and to analyze before investing or expanding the business in this market.
Key Takeaways:
- Understanding the potential market opportunity with precise market size and forecast data.
- Detailed market analysis focusing on the growth of TNF alpha inhibitors
- Factors influencing the growth of the TNF alpha inhibitors
- In-depth competitive analysis of dominant and pure-play vendors.
- Prediction analysis of the TNF alpha inhibitors industry in both developed and developing regions.
- Key insights related to major segments of the TNF alpha inhibitors
- Latest market trend analysis impacting the buying behavior of the consumers.
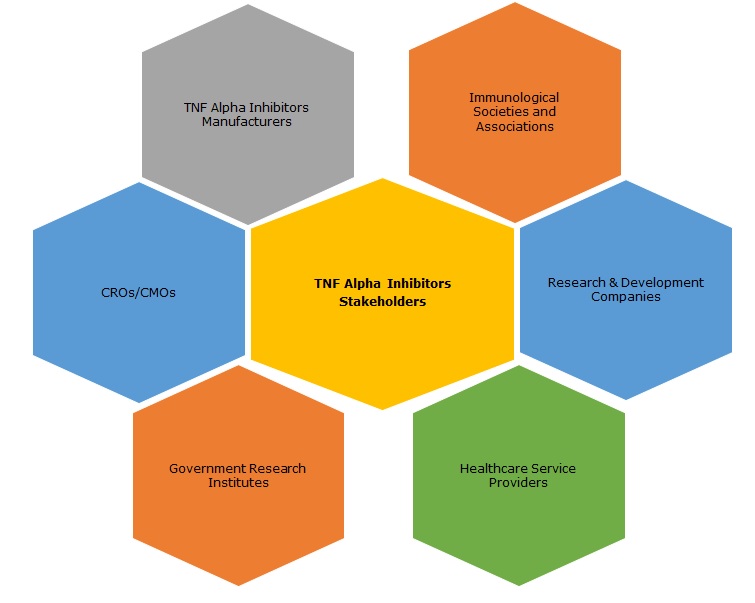
Key Stakeholders:
1 Industry Outlook
1.1 Industry Overview
1.1.1 Global Driver for Pharmaceutical Demand
1.1.2 R&D Pipeline in the Pharmaceutical Industry
1.2 Industry Trends
1.3 Total Addressable Market
1.4 Trends in the Immunotherapy Drugs Market
2 Report Outline
2.1 Report Scope
2.2 Report Summary
2.3 Research Methodology
2.4 Report Assumptions
3 Market Snapshot
3.1 Market Definition – Infoholic Research
3.2 Segmented Addressable Market
3.3 Trends in the TNF Alpha Inhibitors Market
3.4 Related Markets
3.4.1 Oncology Drugs
3.4.2 Biologics
3.4.3 Biosimilars
4 Market Outlook
4.1 Market Segmentation
4.2 PEST Analysis
4.3 Porter 5(Five) Forces
5 Market Characteristics
5.1 DRO – Market Dynamics
5.1.1 Drivers
5.1.1.1 Increasing prevalence of chronic autoimmune diseases
5.1.1.2 Constant research to develop target-based biologics
5.1.2 Opportunities
5.1.2.1 Continuous development of biosimilars
5.1.2.2 Increasing opportunities in the emerging market
5.1.3 Restraints
5.1.3.1 Complex drug development process
5.1.3.2 Patent expiry
5.2 DRO – Impact Analysis
5.3 Key Stakeholders
6 Product: Market Size and Analysis
6.1 Overview
6.2 Adalimumab
6.3 Certolizumab Pegol
6.4 Etanercept
6.5 Golimumab
6.6 Infliximab
7 Pipeline Analysis: Market Size and Analysis
7.1 Overview
7.2 Phase I
7.3 Phase II
7.4 Phase III
8 Regions: Market Size and Analysis
8.1 Overview
8.2 North America
8.2.1 Overview
8.2.2 US
8.2.3 Canada and Others
8.3 Europe
8.3.1 Overview
8.3.2 UK
8.3.3 Germany
8.3.4 Russia and Ukraine
8.3.5 Turkey
8.4 Asia Pacific
8.4.1 Overview
8.4.2 India
8.4.3 Pakistan
8.4.4 Indonesia
8.4.5 Thailand
8.5 Rest of the World
8.5.1 Overview
8.5.2 Mexico
8.5.3 Brazil
8.5.4 Middle East and Africa
9 Competitive Landscape
10 Vendors Profile
10.1 AbbVie Inc.
10.1.1 Overview
10.1.2 Geographic Revenue
10.1.3 Business Focus
10.1.4 SWOT Analysis
10.1.5 Business Strategies
10.2 Novartis AG
10.2.1 Overview
10.2.2 Business Units
10.2.3 Geographic Revenue
10.2.4 Business Focus
10.2.5 SWOT Analysis
10.2.6 Business Strategies
10.3 Amgen Inc.
10.3.1 Overview
10.3.2 Geographic Revenue
10.3.3 Business Focus
10.3.4 SWOT Analysis
10.3.5 Business Strategies
10.4 Johnson & Johnson
10.4.1 Overview
10.4.2 Business Units
10.4.3 Geographic Revenue
10.4.4 Business Focus
10.4.5 SWOT Analysis
10.4.6 Business Strategies
10.5 Celgene Corporation
10.5.1 Overview
10.5.2 Geographic Revenue
10.5.3 Business Focus
10.5.4 SWOT Analysis
10.5.5 Business Strategies
11 Companies to Watch For
11.1 Apogenix
11.1.1 Overview
11.2 Celltrion Healthcare
11.2.1 Overview
11.3 Samsung Bioepis Co. Ltd.
11.3.1 Overview
11.4 3SBio Inc.
11.4.1 Overview
11.5 Shanghai CP Guojian Pharmaceutical Co. Ltd.
11.5.1 Overview
Annexure
Abbreviations
TABLE 1 DRUG CLASS AND THEIR FDA APPROVED INDICATIONS 31
TABLE 2 GLOBAL TNF ALPHA INHIBITOR MARKET REVENUE, BY PRODUCT, 2018–2025 ($MILLION) 32
TABLE 3 DRUG OVERVIEW: ADALIMUMAB 32
TABLE 4 BIOSIMILARS AND NON-ORIGINATOR BIOLOGICALS OF ADALIMUMAB APPROVED OR IN
DEVELOPMENT 34
TABLE 5 DRUG OVERVIEW: CERTOLIZUMAB PEGOL 36
TABLE 6 BIOSIMILARS AND NON-ORIGINATOR BIOLOGICALS OF CERTOLIZUMAB PEGOL IN DEVELOPMENT 37
TABLE 7 DRUG OVERVIEW: ETANERCEPT 38
TABLE 8 BIOSIMILARS AND NON-ORIGINATOR BIOLOGICALS OF ETANERCEPT APPROVED OR
IN DEVELOPMENT 39
TABLE 9 DRUG OVERVIEW: GOLIMUMAB 42
TABLE 10 DRUG OVERVIEW: INFLIXIMAB 43
TABLE 11 BIOSIMILARS AND NON-ORIGINATOR BIOLOGICALS OF INFLIXIMAB IN DEVELOPMENT 45
TABLE 12 MOLECULES IN PIPELINE IN PHASE I 48
TABLE 13 MOLECULES IN PIPELINE IN PHASE II 51
TABLE 14 MOLECULES IN PIPELINE IN PHASE III 54
TABLE 15 GLOBAL TNF ALPHA INHIBITORS DRUGS MARKET REVENUE, BY GEOGRAPHY, 2018–2025
($MILLION) 67
TABLE 16 ABBVIE INC.: OFFERINGS 102
TABLE 17 ABBVIE INC.: RECENT DEVELOPMENTS 102
TABLE 18 NOVARTIS AG: OFFERINGS 107
TABLE 19 NOVARTIS AG: RECENT DEVELOPMENTS 107
TABLE 20 AMGEN INC.: OFFERINGS 115
TABLE 21 AMGEN INC.: RECENT DEVELOPMENTS 115
TABLE 22 JOHNSON & JOHNSON: PRODUCT OFFERINGS 122
TABLE 23 JOHNSON & JOHNSON: RECENT DEVELOPMENTS 122
TABLE 24 CELGENE CORPORATION: OFFERINGS 129
TABLE 25 CELGENE CORPORATION: RECENT DEVELOPMENTS 130
TABLE 26 APOGENIX: SNAPSHOT 134
TABLE 27 APOGENIX: RECENT DEVELOPMENTS 134
TABLE 28 CELLTRION HEALTHCARE: SNAPSHOT 135
TABLE 29 CELLTRION HEALTHCARE: RECENT DEVELOPMENTS 135
TABLE 30 SAMSUNG BIOEPIS CO. LTD.: SNAPSHOT 136
TABLE 31 SAMSUNG BIOEPIS CO. LTD.: RECENT DEVELOPMENTS 136
TABLE 32 3SBIO INC.: SNAPSHOT 138
TABLE 33 3SBIO INC.: RECENT DEVELOPMENTS 138
TABLE 34 SHANGHAI CP GUOJIAN PHARMACEUTICAL CO. LTD.: SNAPSHOT 139
Research Framework
Infoholic Research works on a holistic 360° approach in order to deliver high quality, validated and reliable information in our market reports. The Market estimation and forecasting involves following steps:
- Data Collation (Primary & Secondary)
- In-house Estimation (Based on proprietary data bases and Models)
- Market Triangulation
- Forecasting

Market related information is congregated from both primary and secondary sources.
Primary sources
Involved participants from all global stakeholders such as Solution providers, service providers, Industry associations, thought leaders etc. across levels such as CXOs, VPs and managers. Plus, our in-house industry experts having decades of industry experience contribute their consulting and advisory services.
Secondary sources
Include public sources such as regulatory frameworks, government IT spending, government demographic indicators, industry association statistics, and company publications along with paid sources such as Factiva, OneSource, Bloomberg among others.






