
Orphan Drugs Market Report 2017-2023
- September, 2017
- Domain: Healthcare - Pharmaceuticals
- Get Free 10% Customization in this Report
Global Orphan Drugs Market Orphan Drugs Market-Global drivers, restraints, opportunities, trends and forecasts to 2023 By Drug Types (Biologic, Non-Biologic), Application (Oncology, Metabolic Diseases, Haematology, Infectious Diseases, Neurology, Others) Region (North America, Europe, Asia-Pacific and Rest of the world) - Forecast to 2023
Overview:
Orphan diseases or rare diseases rarely occur among the people, which is nearly 7 out of 10,000 individuals. Around 5,000 to 8,000 rare diseases have been recognized and the origin of approximately 80% of these diseases is from the genes. Regardless of the alleged huge number of individuals being affected by rare life-threatening conditions, the disease burden estimates of the public health are unreliable. About 50% of the orphan genetic ailments are in kids’ with 30% of them cannot live for more than 5 years.
New-born screening, legislation, and construction of national strategies are the major activities being carried out in the orphan drugs market. Legislation has helped in driving the development of more orphan drugs. For instance, the US congress passed Orphan Drug Act in 1983, and it was mainly intended to encourage development of new drugs for rare diseases. Currently, numerous legislation and strategies are developed to facilitate research of orphan drugs globally in more than 35 countries.
Cancer, metabolic diseases, blood diseases, immunologic disease, and neurologic diseases are the types of diseases that are addressed with orphan drugs. Cystic fibrosis, glioma, pancreatic cancer, acute myeloid leukemia, multiple myeloma, renal cell carcinoma, ovarian cancer and Duchenne muscular dystrophy are some of the common indications among the various disease types.
Market Analysis:
The “Global Orphan Drugs Market” is estimated to witness a CAGR of 11.9% during the forecast period 2017–2023. The market is analyzed based on three segments, namely types, applications, and regions.
Regional Analysis:
The regions covered in the report are North America, Europe, Asia Pacific, and Rest of the World (RoW). North America is the leading region for the orphan drugs market growth followed by Europe. Asia Pacific and RoW are set to be the emerging regions. India, China, and Japan are set to be the most attractive destinations due to the large untapped market.
Types Analysis:
The global orphan drugs market by products is segmented into biologics and non-biologics. Biologics orphan drugs is the largest segment in the market with a market share of more than 60% in 2016. It is expected to grow at a double-digit CAGR in the market. However, non-biologic orphan drugs are still expected to hold more than quarter of the market share by 2023. The market is also witnessing various mergers, acquisitions, and collaborations among the top players, which is defining the future of the global orphan drugs market.
Key Players:
Novartis AG, F. Hoffmann-La Roche Ltd, Celgene, Bristol-Myers Squibb Company, Shire pharmaceuticals, Pfizer, Sonafi, and Bayer Healthcare are the key players in the market. These top ten players occupy nearly 50% of the orphan drugs market.
Competitive Analysis:
Currently, large pharmaceutical companies are extremely active in the orphan drugs market. Merger and acquisition is the major trend in the market. For instance, around 40% of the biotechnology companies are acquired between the year 2008-2012, and they have an orphan drug in development. Novartis, GSK, Roche, and Pfizer are the largest orphan drug companies. Pfizer, Gilead, Roche, Shire, BMY, and Celgene are leading orphan drug acquirers. Around 50% of the top 20 orphan drugs were either acquired or in-licensed by large pharmaceutical companies.
Benefits:
The report provides complete details about the usage and adoption rate of orphan drugs during the forecast period and among the regions. With that, key stakeholders can know about the major trends, drivers, investments, vertical player’s initiatives, government initiatives toward the orphan drugs adoption in the upcoming years along with the details of commercial drugs available in the market. Moreover, the report provides details about the major challenges that are going to impact the market growth. Additionally, the report gives complete details about the key business opportunities to key stakeholders to expand their business and capture the revenue in the specific verticals to analyze before investing or expanding the business in this market.
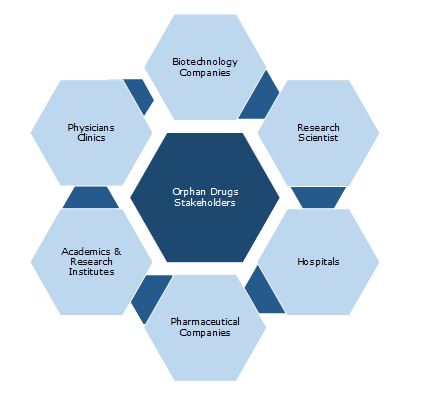
Key Stakeholders:
1 Industry Outlook
1.1 Industry Overview
1.2 Industry Trends
2 Report Outline
2.1 Report Scope
2.2 Report Summary
2.3 Research Methodology
2.4 Report Assumptions
3 Market Snapshot
3.1 Market Definition – Infoholic Research
3.2 Segmented Addressable Market (SAM)
3.2.1 Advantages of Development of Orphan Drug
3.2.2 Trends of orphan drug market
3.3 Related Addressable Markets (RAM)
3.3.1 Oncology (Cancer) Drugs
3.3.2 Active pharmaceutical ingredients (APIs)
3.3.3 Over the counter drugs (OTC)
4 Market Outlook
4.1 Overview
4.2 Regulatory Framework for orphan drugs
4.3 Funding Scenario for orphan drugs
4.4 Market Segmentation
4.5 Porter 5(Five) Forces
4.6 PEST Analysis
5 Market Characteristics
5.1 Market Dynamics
5.1.1 Drivers
5.1.1.1 Conducive government legislation
5.1.1.2 Marketing exclusivity for orphan drugs
5.1.1.3 Growing unmet need for treatment of orphan diseases
5.1.2 Opportunities
5.1.2.1 Growing Novel indications for known orphan drugs
5.1.2.2 Untapped emerging market
5.1.3 Restraints
5.1.3.1 High cost of treatment
5.1.3.2 Lack of Adequate patients for clinical trials
5.1.4 DRO – Impact Analysis
5.1.5 Key Stakeholders
6 Drug Details: Market Size and Analysis
6.1 Overview
7 Types: Market Size and Analysis
7.1 Overview
7.1.1 Biologics
7.1.2 Non-Biologics
8 Application: Market Size and Analysis
8.1 Overview
8.1.1 Oncology
8.1.2 Metabolic Diseases
8.1.3 Hematology
8.1.4 Infectious Diseases
8.1.5 Neurology
8.1.6 Others
9 Pipeline Molecules: Market Size and Analysis
9.1 Overview
10 Regions: Market Size and Analysis
10.1 Overview
10.2 North America
10.2.1 Overview
10.2.2 US
10.2.3 Canada
10.3 Europe
10.3.1 Overview
10.3.1.1 UK
10.3.1.2 Germany
10.3.1.3 France
10.4 APAC
10.4.1 Overview
10.4.1.1 India
10.4.1.2 Japan
10.4.1.3 China
10.5 Rest of the World
10.5.1 Overview
10.5.1.1 Brazil
10.5.1.2 Mexico
10.5.1.3 Africa
10.5.1.4 Middle East
11 Competitive Landscape
12 Vendor Profiles
12.1 Novartis AG
12.1.1 Overview
12.1.2 Business Unit
12.1.3 Geographic Presence
12.1.4 Business Focus
12.1.5 SWOT Analysis
12.1.6 Business Strategy
12.2 F.Hoffmann-La Roche Ltd
12.2.1 Overview
12.2.2 Business Unit
12.2.3 Geographic Presence
12.2.4 Business Focus
12.2.5 SWOT Analysis
12.2.6 Business Strategy
12.3 Celgene Corporation
12.3.1 Overview
12.3.2 Geographic Presence
12.3.3 Business Focus
12.3.4 SWOT Analysis
12.3.5 Business Strategy
12.4 Bristol-Myers Squibb
12.4.1 Overview
12.4.2 Geographic Presence
12.4.3 Business Focus
12.4.4 SWOT Analysis
12.4.5 Business Strategy
12.5 Shire PLC
12.5.1 Overview
12.5.2 Business Unit
12.5.3 Geographic Presence
12.5.4 Business Focus
12.5.5 SWOT Analysis
12.5.6 Business Strategy
13 Companies to Watch For
13.1 Pfizer Inc.,
13.1.1 Overview
13.1.2 Highlights
13.2 Sanofi
13.2.1 Overview
13.3 Bayer AG
13.3.1 Overview
13.4 Alexion Pharmaceutical Inc.
13.4.1 Overview
13.5 Biogen Pharmaceuticals.
13.5.1 Overview
13.6 Eli Lilly and Company.
13.6.1 Overview
13.7 Amgen Inc.
13.7.1 Overview
Annexure
Abbreviations
TABLE 1 REGULATORY FRAMEWORK FOR ORPHAN DRUGS 18
TABLE 2 FUNDED PROJECTS OF 2017 20
TABLE 3 LIST OF ORPHAN DRUGS APPROVED IN THE MARKET 26
TABLE 4 MARKET REVENUE OF TOP 15 DRUGS, 2016 AND 2023 ($BILLION) 44
TABLE 5 MOLECULES IN DEVELOPMENT (2015) 46
TABLE 6 AUTOIMMUNE DISEASES MOLECULES 50
TABLE 7 BLOOD DISORDER MOLECULES 52
TABLE 8 RARE CANCER MOLECULES 52
TABLE 9 BLOOD CANCER MOLECULES 55
TABLE 10 CARDIOVASCULAR DISORDERS MOLECULES 56
TABLE 11 DIGESTIVE DISORDERS MOLECULES 57
TABLE 12 EYE DISORDERS MOLECULES 57
TABLE 13 GENETIC DISORDERS MOLECULES 57
TABLE 14 GROWTH DISORDERS MOLECULES 60
TABLE 15 INFECTIOUS DISORDERS MOLECULES 60
TABLE 16 NEUROLOGIC DISORDERS MOLECULES 61
TABLE 17 RESPIRATORY DISORDERS MOLECULES 62
TABLE 18 TRANSPLANTATION MOLECULES 62
TABLE 19 OTHER DISORDERS MOLECULES IN THE PIPELINE 63
TABLE 20 ORPHAN DRUGS MARKET REVENUE BY REGIONS, 2016–2023 ($BILLION) 65
TABLE 21 TOP 5 ORPHAN DRUGS IN THE US MARKET 66
TABLE 22 REGULATORY AUTHORITIES IN ASIA PACIFIC 69
TABLE 23 NOVARTIS AG: OFFERINGS 76
TABLE 24 NOVARTIS AG: RECENT DEVELOPMENTS 76
TABLE 25 F. HOFFMANN-LA ROCHE LTD.: OFFERINGS 82
TABLE 26 F. HOFFMANN-LA ROCHE LTD.: RECENT DEVELOPMENTS 82
TABLE 27 CELGENE CORPORATION: OFFERINGS 88
TABLE 28 CELGENE CORPORATION: RECENT DEVELOPMENTS 88
TABLE 29 BRISTOL–MYERS SQUIBB: OFFERINGS 92
TABLE 30 BRISTOL–MYERS SQUIBB: RECENT DEVELOPMENTS 92
TABLE 31 SHIRE PLC: OFFERINGS 98
TABLE 32 SHIRE PLC: RECENT DEVELOPMENTS 98
TABLE 33 PFIZER: RECENT DEVELOPMENTS 104
TABLE 34 SANOFI: RECENT DEVELOPMENTS 106
TABLE 35 BAYER AG: RECENT DEVELOPMENTS 107
TABLE 36 ALEXION PHARMACEUTICAL, INC.: RECENT DEVELOPMENTS 108
TABLE 37 BIOGEN PHARMACEUTICALS: RECENT DEVELOPMENTS 109
TABLE 38 ELI LILLY AND COMPANY: RECENT DEVELOPMENTS 110
TABLE 39 AMGEN INC.: RECENT DEVELOPMENTS 111
Research Framework
Infoholic Research works on a holistic 360° approach in order to deliver high quality, validated and reliable information in our market reports. The Market estimation and forecasting involves following steps:
- Data Collation (Primary & Secondary)
- In-house Estimation (Based on proprietary data bases and Models)
- Market Triangulation
- Forecasting

Market related information is congregated from both primary and secondary sources.
Primary sources
Involved participants from all global stakeholders such as Solution providers, service providers, Industry associations, thought leaders etc. across levels such as CXOs, VPs and managers. Plus, our in-house industry experts having decades of industry experience contribute their consulting and advisory services.
Secondary sources
Include public sources such as regulatory frameworks, government IT spending, government demographic indicators, industry association statistics, and company publications along with paid sources such as Factiva, OneSource, Bloomberg among others.






