
eClinical Solutions Market by Product (CDMS, EDC, CTMS, RTMS, Clinical Data Integration Platform, eCOA, Safety Solutions, eTMF, RIMS, and Others), Clinical Trial (Phase I, PhaseII, Phase III, Phase IV), Delivery Mode (Web-hosted, Licensed Enterprise, and Cloud-based), Geography (Asia Pacific, Europe, North America and Rest of the World)- Forecast to 2026
- September, 2020
- Domain: Healthcare - Pharmaceuticals
- Get Free 10% Customization in this Report
eClinical solutions are the ones that combine clinical technology with expertise and are widely used to improve the clinical development process through various data tools such as data management and data analysis. Major factors that are characterizing the market growth are increasing R&D activities by biopharmaceutical and pharmaceutical companies, rising burden of chronic diseases, soaring expenditure on the development of clinical trials, and increased adoption of smart devices for healthcare service management. The reasons for the high demand for eClinical solutions are the rising costs of operational and regulatory requirements.
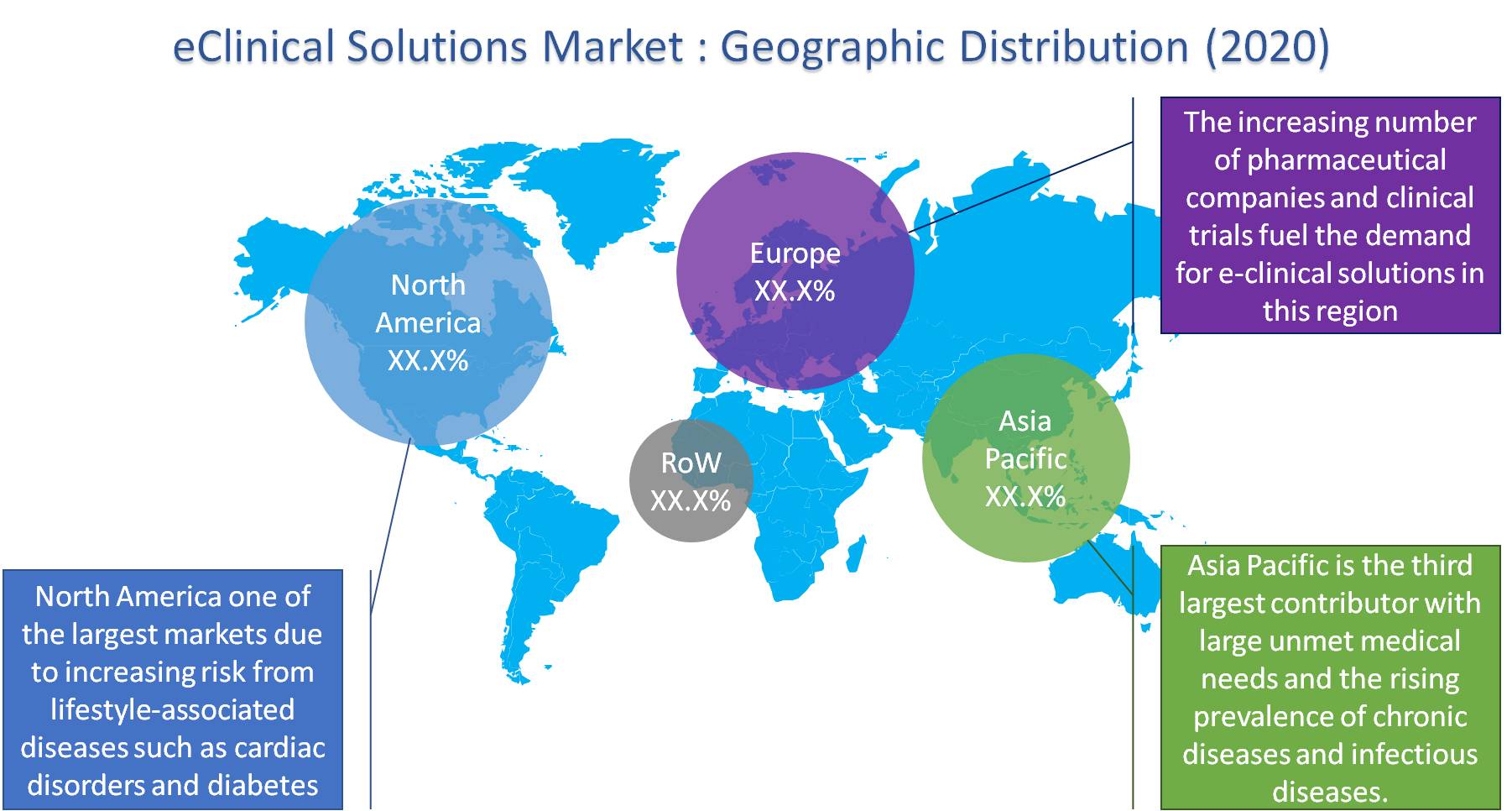
The eClinical solutions market is segregated on the basis of product, clinical trial, delivery mode, and by geography:
Based on Product
- Clinical Data Management Systems (CDMS) and Electronic Data Capture (EDC)
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platform
- Randomization and Trial Supply Management (RTMS)
- Clinical Data Integration Platform
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trial Master Files (eTMF)
- Regulatory Information Management Solutions (RIMS)
- Others (Institutional Review Board Systems, Coding Systems, and Core Lab Integration Solutions)
Based on Clinical Trial
- Phase I
- Phase II
- Phase III
- Phase IV
Based on Delivery Mode
- Web-Hosted
- Licensed Enterprise
- Cloud-Based
Based on Geography:
- North America
- Europe
- Asia Pacific
- Rest of the World
Based on the product, the market segmented by EDC and CDMS, CTMS, Clinical Analytics Platform, RTMS, Clinical Data Integration Platform, eCOA, Safety Solutions, eTMF, RIMS, others. Electronic Clinical Outcome Assessment (eCOA) is assumed to be the rapidly growing segment in the forecast period 2020-2026 since the growing importance of high-quality clinical data. The process of data capturing using eCOA platforms improve the quality of information captured, efficient data collection procedures as well as offers potential data analysis.
Based on the clinical trial phase, the market of EClinical is segmented into Phase I, Phase II, Phase III, and Phase IV. The phase III clinical trial segment is likely to lead the market throughout the forecast period. The need for integration of clinical data management software to restrain overall cost and improve process efficiency as well as an increasing number of drugs successfully reaching phase III is impelling the growth of the segment. Phase III involves the study of the efficacy of a drug by using a group of more than 1,000 patients. A large number of patients increase the complication of the study, which prompts the demand for computer-based systems, thereby rev up the acquisition.
Based on delivery mode, the market has been segmented into three categories: Web-hosted (On-demand), Licensed Enterprise (On-premise), and Cloud-based. Web-hosted solutions have been the dominating segment in the market for eClinical solutions in 2019. The benefits of this segment, such as easy accessibility, usability, and lower investments required, are attributed to the dominance of this segment. Web-hosted products are versatile as they are easily customizable, owing to this fact, providers can also customize the presentation details of information for different user groups. Moreover, these products possess a greater level of interoperability. The segment is predicted to be maintaining its position throughout the forecast period of 2020-2026.
Further, in the end-user segmentation, the market is segmented among the contract research organizations, pharmaceutical, and biopharmaceutical companies, medical device manufacturers, consulting service companies, hospitals, and academic research institutions. The contract research organization is having the highest lucrative share in the market. The segment is expected to enlarge at a distinctive CAGR in the forecast period ascribed to the rising tendency of pharmaceutical companies to decline overall spending. There has been a growth in the adoption of eClinical solutions in R&D, which is further broadening the scope of the segment.
For the year 2019, North America was the leading revenue contributor. The target population, which is constantly increasing, is coming with a risk of lifestyle-associated diseases such as cardiac disorders and diabetes. This is expected to lead to growth in the regional market for EClinical Solutions. Even the presence of significant companies, as well as the attainability of fully-developed infrastructure, is expected to accelerate the growth of the market of eClinical solutions.
Asia Pacific is projected to record a notable CAGR over the 2020-2026 forecast periods. High unfulfilled medical requirements and the increasing incidence of non-communicable diseases such as cancer, cardiovascular, and infectious diseases are stoking the demand for eClinical solutions in the region.
Globally, the EClinical Solutions Market is growing at a CAGR of 12.5% during the forecast period of 2020-2026, with a base value of $6.27 billion in 2020 to $12.76 billion in 2026. The factors which are responsible for driving this market are increasing acquisition of eClinical solutions for advanced data arrangement process as well as the acquisition of novel software solutions, which helps in organizing the research activities in clinical research.
The report also includes the analysis of major players in the area of the eClinical Solutions market. Some of the dominant players consist of Crf Health, Oracle Corporation, Medidata Solutions, Inc, Bioclinica, Inc, Parexel International Corporation, Thermo Fisher Scientific, Inc, Merge Healthcare Incorporated, Omnicomm Systems Inc, ERT CLINICAL, and Maxisit Inc.
This market report will help the market players to comprehend various key market trends, market dynamics, and the needs of the end-users. The qualitative and quantitative analysis of the market definitely enables the user experience of the report.
- Overall information is given by the major competitors on eClinical solutions in this report.
- This research offers present technologies, research activities in the eClinical solutions market.
- Analysis based on the quantitative of the market assists the users to know the actual facts of the market across four major regions, and then accordingly, the company may plan to enter into any region.
1. Executive Summary
2. Industry Outlook
2.1. Industry Overview
2.2. Industry Trends
3. Market Snapshot
3.1. Market Definition
3.2. Market Outlook
3.3. PEST Analysis
3.4. Porter Five Forces
3.5. Related Markets
4. Market characteristics
4.1. Market Evolution
4.2. Market Trends and Impact
4.3. Advantages/Disadvantages of Market
4.4. Regulatory Impact
4.5. Market Offerings
4.6. Market Segmentation
4.7. Market Dynamics
4.7.1. Drivers
4.7.2. Restraints
4.7.3. Opportunities
4.8. DRO - Impact Analysis
5. Product: Market Size & Analysis
5.1. Overview
5.2. Electronic Data Capture and Clinical Data Management Systems
5.3. Clinical Trial Management Systems
5.4. Clinical Analytics Platform
5.5. Randomization and Trial Supply Management
5.6. Electronic Trail Master Files
5.7. Electronic Clinical Outcome Assessment
5.8. Safety Solutions
5.9. Electronic Trail Master Files
5.10. Regulatory Information Management Solutions
5.11. Others
6. Delivery Mode: Market Size & Analysis
6.1. Overview
6.2. Web-hosted
6.3. Licensed Enterprise
6.4. Cloud-based
7. End User: Market Size & Analysis
7.1. Overview
7.2. Pharmaceutical and Biopharmaceutical Companies
7.3. Next-generation Sequencing
7.4. Polymerase Chain Reaction
7.5. Medical Device Manufacturers
7.6. Hospitals
7.7. Academic Research Institutions
8. Clinical Trial Phase: Market Size & Analysis
8.1. Overview
8.2. Phase I
8.3. Phase II
8.4. Phase III
8.5. Phase IV
9. Geography: Market Size & Analysis
9.1. Overview
9.2. North America
9.3. Europe
9.4. Asia Pacific
9.5. Rest of the World
10. Competitive Landscape
10.1. Competitor Comparison Analysis
10.2. Market Developments
10.3. Mergers and Acquisitions, Legal, Awards, Partnerships
10.4. Product Launches and execution
11. Vendor Profiles
11.1. Oracle Corporation
11.1.1. Overview
11.1.2. Product Offerings
11.1.3. Geographic Revenue
11.1.4. Business Units
11.1.5. Developments
11.1.6. Business Strategy
11.2. Medidata Solutions, Inc
11.2.1. Overview
11.2.2. Product Offerings
11.2.3. Geographic Revenue
11.2.4. Business Units
11.2.5. Developments
11.2.6. Business Strategy
11.3. Parexel International Corporation
11.3.1. Overview
11.3.2. Product Offerings
11.3.3. Geographic Revenue
11.3.4. Business Units
11.3.5. Developments
11.3.6. Business Strategy
11.4. Bioclinica, Inc.
11.4.1. Overview
11.4.2. Product Offerings
11.4.3. Geographic Revenue
11.4.4. Business Units
11.4.5. Developments
11.4.6. Business Strategy
11.5. Thermo Fisher Scientific, Inc
11.5.1. Overview
11.5.2. Product Offerings
11.5.3. Geographic Revenue
11.5.4. Business Units
11.5.5. Developments
11.5.6. Business Strategy
11.6. Crf Health.
11.6.1. Overview
11.6.2. Product Offerings
11.6.3. Geographic Revenue
11.6.4. Business Units
11.6.5. Developments
11.6.6. Business Strategy
11.7. ERT CLINICAL
11.7.1. Overview
11.7.2. Product Offerings
11.7.3. Geographic Revenue
11.7.4. Business Units
11.7.5. Developments
11.7.6. Business Strategy
11.8. Merge Healthcare Incorporated
11.8.1. Overview
11.8.2. Product Offerings
11.8.3. Geographic Revenue
11.8.4. Business Units
11.8.5. Developments
11.8.6. Business Strategy
11.9. Omnicomm Systems, Incs.
11.9.1. Overview
11.9.2. Product Offerings
11.9.3. Geographic Revenue
11.9.4. Business Units
11.9.5. Developments
11.9.6. Business Strategy
11.10. Maxisit Inc
11.10.1. Overview
11.10.2. Product Offerings
11.10.3. Geographic Revenue
11.10.4. Business Units
11.10.5. Developments
11.10.6. Business Strategy
12. Companies to Watch
12.1. Aris Global
12.1.1. Overview
12.1.2. Market
12.1.3. Business Strategy
12.2. Advarra
12.2.1. Overview
12.2.2. Market
12.2.3. Business Strategy
12.3. Eclinicalworks
12.3.1. Overview
12.3.2. Market
12.3.3. Business Strategy
12.4. Mastercontrol, Inc.
12.4.1. Overview
12.4.2. Market
12.4.3. Business Strategy
12.5. Resonance Health
12.5.1. Overview
12.5.2. Market
12.5.3. Business Strategy
13. Analyst Opinion
14. Annexure
14.1. Report Scope
14.2. Market Definitions
14.3. Research Methodology
14.3.1. Data Collation and In-house Estimation
14.3.2. Market Triangulation
14.3.3. Forecasting
14.4. Report Assumptions
14.5. Declarations
14.6. Stakeholders
14.7. Abbreviations
TABLE 1. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 2. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 3. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 4. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR CLINICAL ANALYTICS PLATFORM, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 5. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR ELECTRONIC TRAIL MASTER FILES, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 6. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 7. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR OTHERS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 8. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR SAFETY SOLUTION, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 9. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR ELECTRONIC TRAIL MASTER FILES, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 10. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR REGULATORY INFORMATION MANAGEMENT SOLUTIONS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 11. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR OTHERS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 12. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 13. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR WEB-HOSTED, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 14. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR LICENSED ENTERPRISE, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 15. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR CLOUD-BASED, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 16. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR END USER, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 17. GLOBAL ECLINICAL SOLUTIONSMARKET VALUE FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 18. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR CONTRACT RESEARCH ORGANIZATIONS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 19. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR CONSULTING SERVICE COMPANIES, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 20. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR MEDICAL DEVICE MANUFACTURERS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 21. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR HOSPITALS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 22. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR ACADEMIC RESEARCH INSTITUTIONS, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 23. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR CLINICAL TRIAL PHASE, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 24. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR PHASE I, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 25. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR PHASE II, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 26. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR PHASE III, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 27. GLOBAL ECLINICAL SOLUTIONS MARKET VALUE FOR PHASE IV, BY GEOGRAPHY, 2020-2026 (USD MILLION)
TABLE 28. NORTH AMERICA ECLINICAL SOLUTIONSMARKET VALUE, BY COUNTRY, 2020-2026 (USD MILLION)
TABLE 29. NORTH AMERICA ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 30. NORTH AMERICA ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 31. NORTH AMERICA ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 32. U.S ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 33. U.S ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 34. U.S ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 35. CANADA ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 36. CANADA ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 37. CANADA ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 38. EUROPE ECLINICAL SOLUTIONSMARKET VALUE, BY COUNTRY, 2020-2026 (USD MILLION)
TABLE 39. EUROPE ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 40. EUROPE ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 41. EUROPE ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 42. ASIA PACIFIC ECLINICAL SOLUTIONSMARKET VALUE, BY COUNTRY, 2020-2026 (USD MILLION)
TABLE 43. ASIA PACIFIC ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 44. ASIA PACIFIC ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 45. ASIA PACIFIC ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 46. REST OF WORLD ECLINICAL SOLUTIONSMARKET VALUE, BY COUNTRY, 2020-2026 (USD MILLION)
TABLE 47. REST OF WORLD ECLINICAL SOLUTIONSMARKET VALUE, BY PRODUCT, 2020-2026 (USD MILLION)
TABLE 48. REST OF WORLD ECLINICAL SOLUTIONSMARKET VALUE, BY DELIVERY MODE, 2020-2026 (USD MILLION)
TABLE 49. REST OF WORLD ECLINICAL SOLUTIONSMARKET VALUE, BY CLINICAL TRIAL PHASE, 2020-2026 (USD MILLION)
TABLE 50. ORACLE CORPORATION.: OVERVIEW
TABLE 51. ORACLE CORPORATION.: STRATEGIC SNAPSHOT
TABLE 52. ORACLE CORPORATION.: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 53. ORACLE CORPORATION.: PRODUCT/SERVICE PORTFOLIO
TABLE 54. MEDIDATA SOLUTIONS, INC: OVERVIEW
TABLE 55. MEDIDATA SOLUTIONS, INC: STRATEGIC SNAPSHOT
TABLE 56. MEDIDATA SOLUTIONS, INC: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 57. MEDIDATA SOLUTIONS, INC: PRODUCT/SERVICE PORTFOLIO
TABLE 58. PAREXEL INTERNATIONAL CORPORATION: OVERVIEW
TABLE 59. PAREXEL INTERNATIONAL CORPORATION: STRATEGIC SNAPSHOT
TABLE 60. PAREXEL INTERNATIONAL CORPORATION: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 61. PAREXEL INTERNATIONAL CORPORATION: PRODUCT/SERVICE PORTFOLIO
TABLE 62. BIOCLINICA, INC.: OVERVIEW
TABLE 63. BIOCLINICA, INC.: STRATEGIC SNAPSHOT
TABLE 64. BIOCLINICA, INC.: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 65. BIOCLINICA, INC.: PRODUCT/SERVICE PORTFOLIO
TABLE 66. THERMO FISHER SCIENTIFIC, INC: OVERVIEW
TABLE 67. THERMO FISHER SCIENTIFIC, INC: STRATEGIC SNAPSHOT
TABLE 68. THERMO FISHER SCIENTIFIC, INC: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 69. THERMO FISHER SCIENTIFIC, INC: PRODUCT/SERVICE PORTFOLIO
TABLE 70. Crf Health.: OVERVIEW
TABLE 71. Crf Health.: STRATEGIC SNAPSHOT
TABLE 72. Crf Health.: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 73. Crf Health.: PRODUCT/SERVICE PORTFOLIO
TABLE 74. ERT CLINICAL: OVERVIEW
TABLE 75. ERT CLINICAL: STRATEGIC SNAPSHOT
TABLE 76. ERT CLINICAL: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 77. ERT CLINICAL: PRODUCT/SERVICE PORTFOLIO
TABLE 78. MERGE HEALTHCARE INCORPORATED.: OVERVIEW
TABLE 79. MERGE HEALTHCARE INCORPORATED.: STRATEGIC SNAPSHOT
TABLE 80. MERGE HEALTHCARE INCORPORATED.: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 81. MERGE HEALTHCARE INCORPORATED.: PRODUCT/SERVICE PORTFOLIO
TABLE 82. OMNICOMM SYSTEMS, INCS.: OVERVIEW
TABLE 83. OMNICOMM SYSTEMS, INCS.: STRATEGIC SNAPSHOT
TABLE 84. OMNICOMM SYSTEMS, INCS.: BUSINESS OPPORTUNITIES AND OUTLOOK
TABLE 85. OMNICOMM SYSTEMS, INCS.: PRODUCT/SERVICE PORTFOLIO
TABLE 86. MAXISIT INC.: OVERVIEW
TABLE 87. MAXISIT INC.: STRATEGIC SNAPSHOT
TABLE 88. MAXISIT INC.: BUSINESS OPPORTUNITIES AND OUTLO
TABLE 89. MAXISIT INC.: PRODUCT/SERVICE PORTFOLIO
Research Framework
Infoholic Research works on a holistic 360° approach in order to deliver high quality, validated and reliable information in our market reports. The Market estimation and forecasting involves following steps:
- Data Collation (Primary & Secondary)
- In-house Estimation (Based on proprietary data bases and Models)
- Market Triangulation
- Forecasting

Market related information is congregated from both primary and secondary sources.
Primary sources
Involved participants from all global stakeholders such as Solution providers, service providers, Industry associations, thought leaders etc. across levels such as CXOs, VPs and managers. Plus, our in-house industry experts having decades of industry experience contribute their consulting and advisory services.
Secondary sources
Include public sources such as regulatory frameworks, government IT spending, government demographic indicators, industry association statistics, and company publications along with paid sources such as Factiva, OneSource, Bloomberg among others.






